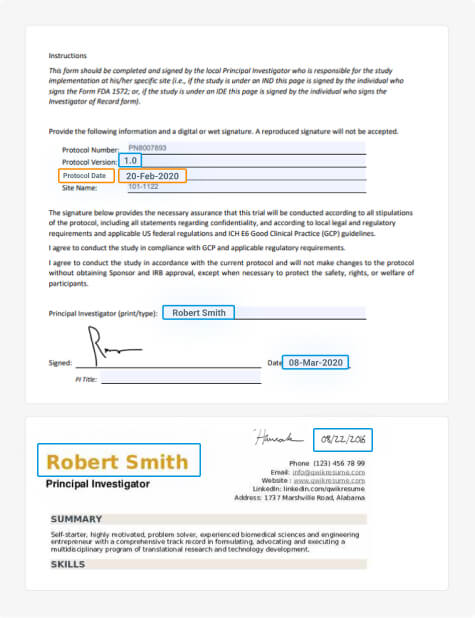
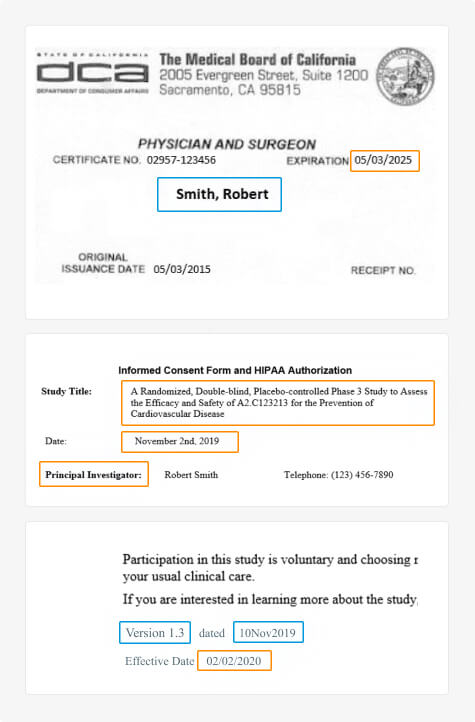
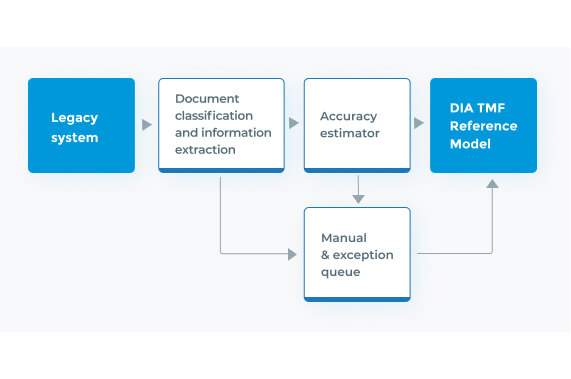
Clinical Trial Master File Migration & Information Extraction
Fast, accurate, and consistent migration and information extraction of large-scale clinical trial documents from unstructured or legacy systems to the DIA TMF.
80%
Time and labor savings