LOGICAL OBSERVATION IDENTIFIERS, NAMES, and CODES (LOINC)
Logical Observation Identifiers, Names, and Codes (LOINC) is a classification for clinical observations. It is primarily used for lab results but can also be applied to aspects of a physical examination or any other clinical observation.
LOINC development was initiated at The Regenstrief Institute, Indianapolis and the LOINC committee (initiated in 1994). It started as an independent consortium, led by Clement J. McDonald and Stanley M. Huff.
They have developed a coding system for tests and observations. Originally called Laboratory Observations, Identifiers, Names and Codes (LOINC).
Their system was then upgraded to include nonlaboratory observations (like vital signs and electrocardiograms). Hence, the name was accordingly upgraded to suit the changes and the term “Laboratory” was replaced by “Logical”. Currently, the clinical portion of LOINC includes vital signs, echocardiograms, gastrointestinal endoscopy, EKGs and many others.
The main target of establishing the LOINC is to ensure a smooth electronic transfer of clinical data from laboratories (data source) to hospitals/physician’s offices, or payers (data destination).
LOINC is designed to be compatible with HL7 messaging standard.
LOINC database has 41,000 observation terms, where almost 31,000 of them are related to laboratory testing code (chemistry, hematology, serology, microbiology, and toxicology).
In the US, famous labs such as Quest and LabCorp have matched their internal codes to LOINC.
Understanding a LOINC Record
Each record of the LOINC database contains six different axes. The axes are colon-delimited. The six axes can be explained in brief as follows:
1- Component: What is meant to be measured.
2- Property: measurement characteristics (e.g.: concentration, numeric fraction, …etc).
3- Timing: point in time when the measurement was completed.
4- System: Where the component originates.
5- Scale: How the results will be reported.
6- Method (optional): e.g.: qualitative or quantitative, automatic or manual, …etc.
A LOINC code example is 5792-7, Glucose:MCNC:PT:Urine:QN:Teststrip, is a quantitative test that measures glucose amount in the urine.
LOINC Files
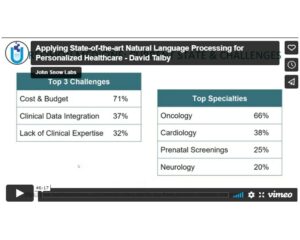
If you care about obtaining clean and well-reviewed data in standardized formats, John Snow Labs catalog can be an ideal repository for you. It contains a full LOINC Data Package. This data package includes multiple datasets includes many valuable datasets related to drug terminologies. It includes datasets for many valuable datasets related to LOINC, like LOINC Identifiers Names And Codes, LOINC Names Codes Map To Source Organization, and LOINC Names And Codes Source Organization.
RELMA®
A mapping assistant to assist mapping of internal test codes to LOINC codes. It is a Windows-based program designed to support browsing and searching the LOINC database and mapping local test codes to LOINC codes.
LOINC and RELMA are available here free of charge.
LOINC Community
The most interesting thing about LOINC is that you discover during your readings that it exceeded the idea of being just a standard for controlled vocabulary. LOINC has a broad community.
LOINC has been applied and adopted in the USA, Canada, Australia, New Zealand, Estonia, Germany, Switzerland, Brazil, and Korea. Currently, it is available in (simplified) Chinese and soon will be available in German and Spanish.
You can be part of this community by registering for a free user account on the LOINC.org website. You can then receive an invitation to participate in their surveys and accordingly receive the results of those surveys and also you can follow the LOINC events or register to attend their Workshops and Public Committee Meetings.
EHR-Lab Interoperability and Connectivity Standards (ELINCS)
ELINCS was introduced in 2005 and soon California Healthcare Foundation became a sponsor for it and HL7 incorporated it in its plans for adoption and maintenance.
It acts as a lab interface for ambulatory EHRs. Moreover, it has an important role as a “constraint” or refinement of HL7.
Labs daily workflow usually entails requests submission and receiving the results. The requests are submitted from the physician office to the lab and the results are sent back from the lab to the physician office. The messaging process almost takes place through fax or email where the results are added later to the EHR of the patient.
Such type of messaging that ends with data to be entered into the EHRs needs standardization in the format and the coding system (like LOINC).
Nowadays, for a hospital to be EHR certified, this hospital must satisfy the Certification Commission for Healthcare Information Technology (CCHIT) requirements for ELINCS standards.
By leveraging Generative AI in Healthcare and the power of a Healthcare Chatbot, healthcare providers can overcome big data challenges and improve the accuracy and efficiency of laboratory data processing, ensuring better patient outcomes.