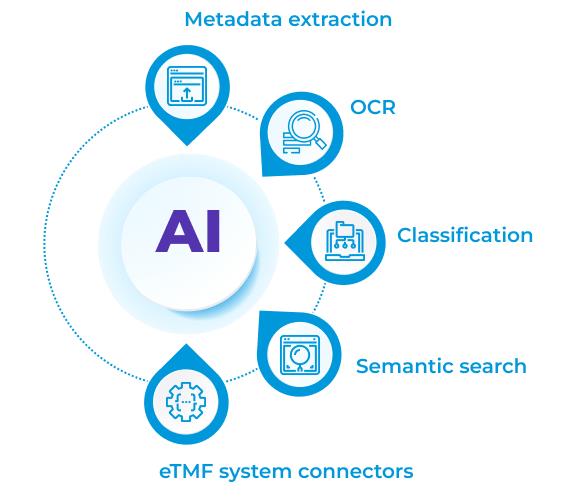
Clinical trials are among the most time-consuming administrative endeavors in the business world. Any technology that enables these trials to be conducted more efficiently while improving their quality is wholeheartedly welcomed. And this is the reason for the excitement surrounding the electronic trial master file (eTMF). eTMF is one of the several areas requiring a significant amount of manual effort for collecting and classifying documentation used to verify Good Clinical Practice (GCP) compliance. Though a time-consuming back-office task, it is a must for regulatory compliance throughout clinical research. This article analyses the issues surrounding the current eTMF and explains how a new model can address them by using Artificial Intelligence (AI).
Shortcomings of current eTMF
eTMF is mandatory for regulatory compliance and a yardstick to measure GCP. However, the humungous human hours spent compiling cause several issues. Some of them are:
Misclassified documents and duplicates
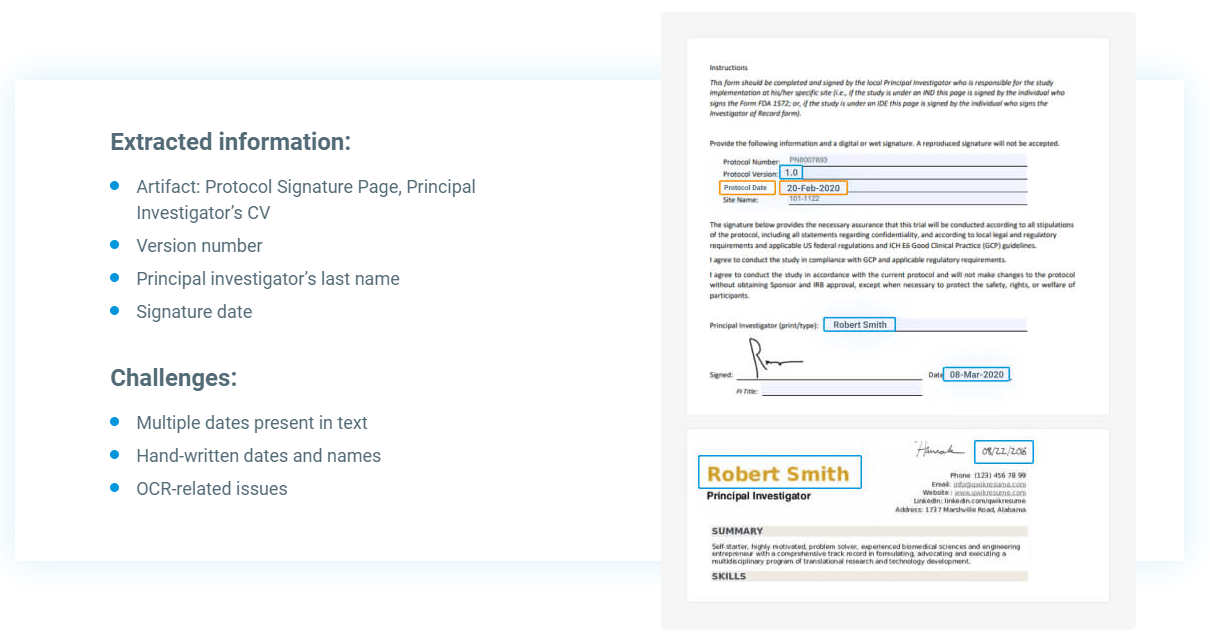
A single clinical experiment can generate thousands of records. These papers must be classified by kind and critical properties or metadata extracted. Professionals must also enhance the reports manually to ensure that the documents are suitable for their defined purposes and accurately mapped. All these tasks consume a significant amount of time and effort. Human errors frequently cause a mismatch between extracted metadata and document content and increase the number of duplicates, leading to poor eTMF quality.
Poor search algorithms
The documents are essential since they allow for the evaluation of a clinical trial and the quality of the data. Keyword searches only return records with the keywords in the title and not the document body. Search results often do not represent the operator’s purpose, which compromises the quality of the data provided.
Missed deadlines
A missing alert function and the inability to monitor papers’ filing and finalization on time result in a long waiting period. It leads to further delay in the pipeline as one has to check whether the documents get finalized every time.
Increased compliance risks
Frustration surrounding document quality control is often raised around conference round tables worldwide, with many clinical teams still bearing the burden of guaranteeing eTMF content quality. Due to a lack of understanding about why documents fail QC and how frequently they should be resubmitted and updated, compliance risks increase.
Non-standardization led errors
Another element contributing to eTMF complexity is the presence of thousands of documents or artifacts from departments other than clinical operations. These artifacts could be in various formats, such as Word, PDF, scanned PDFs, Excel with automatic formulae evaluations, and even PowerPoint presentations. Non-standardization makes document compilation and comparison harder. As they contain a lot of unstructured, i.e., free text data, it’s significantly more difficult to swiftly classify them or extract meaningful information from them at scale, which increases the possibility of human error.
Advantages of eTMF add-on
An automated solution is required to eliminate wasteful and inefficient manual labor and improve output consistency and repeatability. However, robotic process automation (RPA) is not the solution. RPA bots are not intelligent enough to interpret unstructured data in clinical trial records. Only an AI and NLP-based solution can assist clinical trial investigators, sponsors, and other stakeholders in efficiently addressing all the difficulties mentioned above. The AI and NLP-powered Electronic Trial Master File Migration System offer an organized, fast, repeatable, and scalable method of processing eTMF artifacts. The following are the advantages of the eTMF add on:
Saving labor effort
Precise user assistance reduces training expenses, while the simple operation supported by processes speeds up data entry. AI solutions are speeding up document filing and cutting the cost of manual processing. What AI excels at are things that humans find tedious and repetitious. When you can use AI to perform those activities, you can free up your employees to do work requiring more human effort.
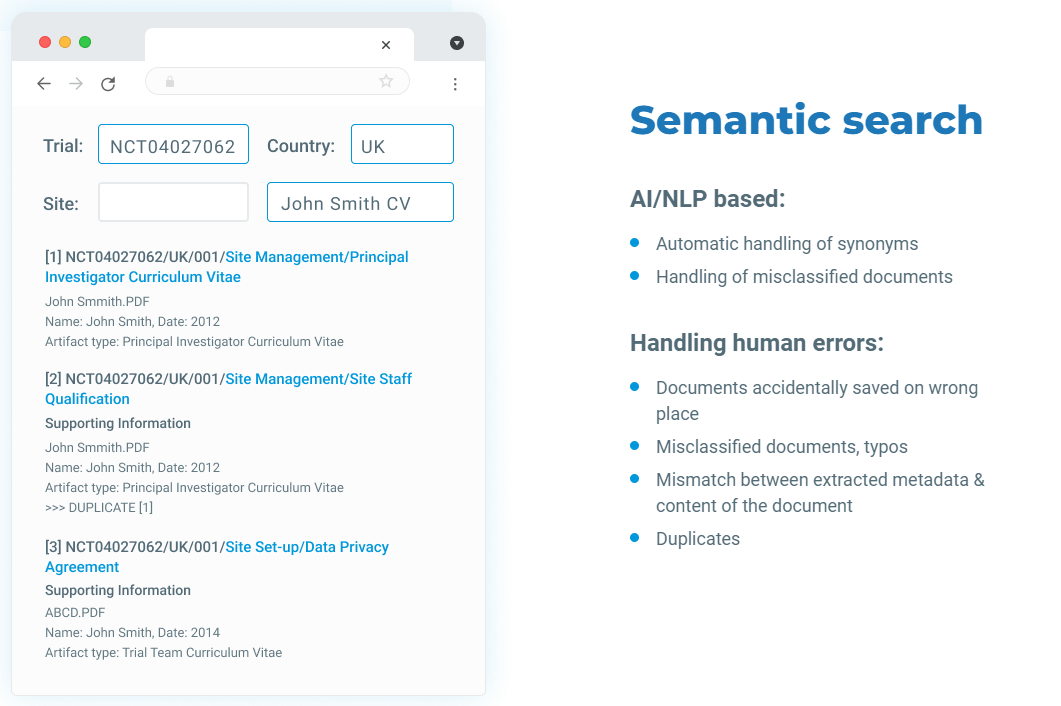
Semantic search
A search engine based on natural language processing and text mining can discover facts, relationships, and claims in texts and deliver relevant information to answer questions. Such search engines can comprehend several linguistic representations of a single notion and extract the correct information you require to answer queries, making the process easier and simpler.
Advanced reporting
You can quickly generate advance reports based on any data collected in the system by using a powerful search engine based on various characteristics found in the file name. By doing so, the researcher can narrow their search to the area they want to explore. Thus, the user does not need to specify all filter requirements at once.
Real-time document tracking and viewing
Monitor the status of documents as soon as they arrive in eTMF. Also, it reduces the chances of data entry errors, allowing the workflow to continue uninterrupted. It enables monitors to obtain data in seconds and as frequently as required. By using less time, the monitor will be able to focus their attention on other parts of the clinical trials, resulting in higher quality studies.
Improved eTMF metrics
Not having access to reliable, improved eTMF metrics throughout a clinical trial is like driving at night with your headlights turned off. Dashboards, metrics, and KPIs provide rapid visibility into eTMF and lead users through addressing any issues that may arise to increase process efficiencies. You can examine the workflow metadata to see which artifacts are usually erroneously indexed and the reasons for rejecting other documents.
Document classification powered by AI
Determine the document type using a predefined classification scheme, such as the TMF Reference Model. Documents are automatically categorized; human inspection is only required to recheck artifacts, sub-artifacts, and file types. It increases speed and quality by introducing a more effective eTMF strategy and automated procedures and enabling continuous improvement. All these additions allow for the creation of a higher-quality eTMF.
Conclusion
Massive amounts of data are generated from the beginning until the end of a clinical trial. Trial investigators, sponsors, and monitors frequently need to categorize and extract critical information from these records to help downstream procedures and decision-making. However, manual data processing becomes neither efficient nor sustainable as document volumes grow. As a result, automation is essential. However, the majority of automated solutions only function with structured data. They are unable to analyze unstructured data in clinical trial paperwork. This add-on significantly increases eTMF processing speeds and output quality over manual techniques, thanks to its advanced automation capabilities and machine learning algorithms trained on millions of eTMF documents.